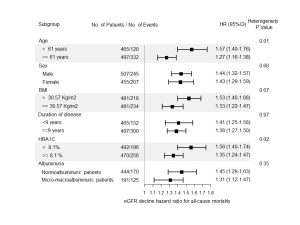
Background and hypothesis: The independent role of glomerular filtration rate (GFR) decline in shaping the risk of mortality in people with type 2 diabetes has only been partially addressed. We investigated the association between changes of estimated GFR (eGFR) over time and the risk of all-cause mortality in people with type 2 diabetes from Southern Italy. We also investigated whether renal dysfunction mediates the effect of tryptophan metabolism on death risk. Methods: The study was carried out in 962 patients with type 2 diabetes, from the aggregate Gargano Mortality Study (aGMS), who had at least three eGFR recordings and at least 1.5 years of follow-up. To investigate whether the eGFR annual decline acts as a mediator of the relationship between mortality risk and tryptophan metabolism, by means of serum kynurenine-to-tryptophan ratio (KTR), a causal mediation analysis was performed in a representative subgroup of 575 individuals. Results: Age and sex adjusted annual incident rate of mortality was 2.75 events per 100 person-years. The median annual rate of decline of eGFR was 1.3 ml/min per 1.73 m2 per year (range -3.7;7.8). A strong association was observed between the annual rate of eGFR decline and mortality: for each ml/min increase in the annual rate of eGFR loss, there was a 31% increase in all-cause mortality, even after adjustment for several confounders [adjusted HR 1.31 (1.21-1.41), p<0.001]. In subgroup analyses, the association between eGFR decline and mortality, was significantly stronger in individuals relatively younger and with better glycaemic control (Fig. 1). KTR was associated with both eGFR decline (R2=0.16, p=0.0002) and all-cause mortality (adjusted HR per 1 SD increase=1.33, 95% CI=1.09-1.63). Causal mediation analysis showed that a significant proportion (i.e., 24.3%, p=0.006) of the association between KTR and mortality was mediated by eGFR decline. Conclusion: In patients with type 2 diabetes, eGFR decline is independently associated with risk of all-cause mortality and mediates approximately one fourth of the association between tryptophan metabolism and death. Evidence of a pathway that starts from tryptophan metabolism and through renal dysfunction gets to the risk of death opens the way for the search for new tailor-made treatments in patients with specific metabolic signatures.