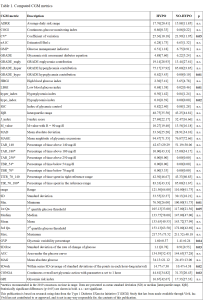
Background and aims: Exercise is recommended in people with type 1 diabetes (T1D), but risk of hypoglycemia – which may occur even hours following exercise (especially overnight) – adversely affects their involvement in it. Our aim was to evaluate which CGM metrics assessed before exercise starting are more predictive of hypoglycemia occurrence. Methods. 51 young subjects with T1D, performing a free-daily-living running exercise session, were selected from the T1DEXI-P dataset, average age being 13.7±1.7 years, with 18 females and 33 males and average HbA1c of 6.8±1.1% (mean±standard deviation, SD). All selected subjects were on insulin pump/closed loop therapy and worn a Dexcom G6 CGM device. Each CGM recording was assigned to HYPO (n=30) or NO-HYPO (n=21) group, if hypoglycemia was detected or not during the period ranging from start of the exercise session until the following morning, respectively. Pre-exercise CGM data (defined as the interval from 5 hours before to exercise starting) were used to extract a total of 40 CGM metrics (Tab. 1). Results: 10 out of the 40 CGM metrics resulted significantly different between the two groups (p≤0.05). The HYPO group showed significantly increased glycemic variability (as for CV, GVP, SD.Roc, SDwsh, CONGA: see Table 1) and increased hypoglycemia indexes (as for GRADE_hypo, LBGI, hypo_index), together with lower minimum glucose and a tendency to significantly lower values of 1st quartile glucose threshold (1st Qu). Of note, among the glycemic variability metrics, CV (one of the metrics recommended by international consensus) showed borderline significant difference, while relatively strong significance was shown for SDwsh, thus highlighting that when a short CGM window is considered metrics other than those recommended in the consensuses may be relevant. Conclusions: The CGM metrics evaluated in a 5-hours pre-exercise window may have a role in predicting upcoming hypoglycemia in pediatric patients with T1D.