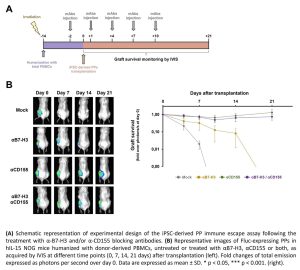
To date, the clinical application of human induced pluripotent stem cells (iPSCs)-derived β cells for the treatment of Type 1 Diabetes (T1D) is still hindered by immune-mediated response. Stem cell-derived pancreatic progenitors (PPs) entered in phase I/II clinical trials as they are characterized by null expression of MHC-I. Although the absence of MHC-I molecules avoids the T cell response, it can trigger Natural Killer (NK) cells via missing-self recognition. Our previous investigations highlighted the role of the immune checkpoints B7-H3 and CD155 in recognition of MHC-I-null cells by NKs. Since during pancreatic differentiation a strong down-modulation of MHC-I surface expression occurs, iPSC-derived PPs may result in higher susceptibility to NKs recognition and killing. Therefore, we aimed at assessing engraftment and survival of iPSC-derived PPs after transplantation into humanized mice treated with monoclonal antibodies (mAbs) antagonizing the human B7-H3 and/or CD155 ligands. We generated luciferase-expressing human iPSCs and differentiated them into PDX1+/NKX6-1+ PPs. We transplanted the iPSC-derived PPs in hindlimb muscles of hIL-15 NOG mice humanized with donor-derived PBMCs. Graft survival was evaluated up to 21 days by in vivo imaging system. Mice were treated or not with five injections of 1.25 mg/Kg of α-B7-H3, 2,5 mg/Kg of α-CD155, or both mAbs every 3 days. The hIL-15 NOG mouse model ensured persistence of both CD4+ and CD8+ T lymphocytes, as well as of hCD56brightCd16- (precursor) and CD56dimCD16+ (effector) NK cells, over 28 days upon injection of hPBMCs. We confirmed that iPSC-derived PPs are rejected in untreated humanized mice within one week after transplantation, compared to the mAb-treated groups (p<0.001). Treatment with α-B7-H3 alone did not confer protection against immune recognition, as we observed a progressive decrease of bioluminescence signal, and the graft was rejected between 14 and 21 days after transplantation. Conversely, in humanized mice treated with α-CD155 alone or in combination with α-B7-H3, the transplanted PPs survived up to 21 days (p<0.001) and properly differentiated in vivo into endocrine islets. We demonstrated that blocking mAbs against B7-H3 and CD155 axes can be exploited to increase the immune compatibility of iPSC-derived pancreatic derivatives without lasting genetic manipulation, thus increasing chances for future use in therapy of T1D.