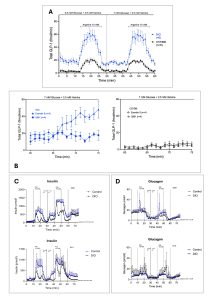
Background: It has been recently hypothesized that alpha cells are a potential source of intra-islet GLP-1, eventually exerting paracrine effects on islet function. Further, glucagon receptor knockout mice exhibit significant pancreatic 7-36 GLP-1 amide secretion and expression. However, it is still unknown whether intraislet GLP-1 production and secretion is modulated by insulin resistance and glucose dysmetabolism. In this study, we aim to explore the impact of insulin resistance on intra-islet proglucagon-derived peptides in a population of Diet-Induced Obese (DIO) mice. Methods: we performed a 75 min pancreatic perfusion on n=8 DIO vs n=8 C57BL/6 control mice at low (3.5 mM) and medium (7 mM) glucose concentrations + 3.5 mM Vamine and 10 mM arginine stimulation; at the end, we infused insulin receptor antagonist S961 (n=4) or GLP-1 receptor antagonist Exendin 9 (n=4) to investigate possible involvement of insulin and GLP-1 receptor signaling. Total GLP-1 levels were measured using RIA; Insulin and Glucagon levels were measured through commercial ELISA kit (Mercodia). Results: Compared to controls, DIO mice showed a significant secretion of total GLP-1 overtime during perfusions (p<0.0001), with no differences between 3.5 and 7 mM glucose and with a preserved response to arginine (Fig. A). Further, S961 did not affect total GLP-1 secretion, whereas Exendin 9 led to increased intra-islet total GLP-1 secretion in DIO mice, as in a positive feedback-control mechanism (Fig. B). As expected, DIO mice showed increased insulin secretion at 7 mM glucose and during arginine stimulation (Fig. C), while no differences were observed in the glucagon secretion (Fig. D). S961 and Exendin 9 did not affect insulin nor glucagon secretion in the 2 groups. Conclusions: our data demonstrate that intraislet GLP-1 secretion is significantly increased in a rodent model of obesity and insulin resistance. In particular, this secretion pattern seems to be regulated by the activity of GLP-1 receptor itself. Further studies will be necessary to clarify the pathophysiologic action of intrapancreatic GLP-1 on insular environment and the potential pharmacological implications of these findings.