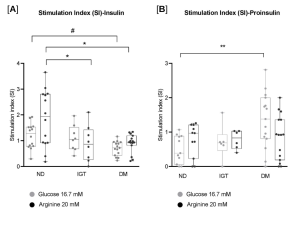
Pancreatic islet isolation is critical for type 2 diabetes research. Although –omics approaches have shed light on islet molecular profiles, inconsistencies persist; on the other side, functional studies are essential, but they require reliable and standardized isolation methods. Here, we propose a simplified protocol applied to very small-sized samples (1-1.5 g of weight) collected from partially pancreatectomized living donors, whose β-cell functional parameters had been thoroughly characterized before surgery through different kinds of metabolic tests. Islet isolation was performed digesting pancreatic surgical specimens using a collagenase P solution and a shaking water bath, followed by Lympholyte density gradient separation. Islets were finally stained with dithizone and functionally assessed with low/high glucose (3.3 mM and 16.7 mM) and arginine (20 mM), in order to evaluate insulin and proinsulin secretion. Importantly, ex vivo secretion mirrored the donors’ metabolic profiles: insulin release significantly decreased in diabetic islets compared to non-diabetic islets; conversely, proinsulin showed an increasing trend from non-diabetic to diabetic islets (see panel [A] and [B]; # p<0.01;* p<0.05 in [A]; ** p<0.01 in [B]). Thus, this novel islet isolation method enables the generation of islet preparations closely reflecting donors’ clinical profiles, simplifying the isolation process and eliminating the need for a Ricordi chamber. Moreover, such deep in vivo characterization has not been reported in other islet isolation studies. This offers a valuable opportunity for targeted-study islet physiology and for new personalized pharmacological approaches.