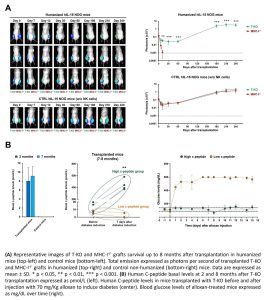
Human Natural Killer (NK) cells represent an heterogenous population of lymphocytes that regulate several aspects of the alloimmune response in solid organ transplantation. Reactivity of NK cells depends on the specific ligands expressed on target cells. Recently, MHC-I-/- stem cell derived β-cells have been proposed as alternative, potentially unlimited source for the treatment of Type 1 Diabetes (T1D). Although MHC-I abrogation via beta-2-microglobulin (B2M) knock-out prevents T cell attack, it triggers NK cells via missing self-recognition, leading to graft loss. As we have demonstrated that the NK activating ligands CD276 and CD155 mediate MHC-I-null killing, we propose their knock-out to escape NK and ensure long-term survival and function of transplanted β-cells in diabetic humanized mice. We generated luciferase-expressing wild type (WT), MHC-I-/- and B2M/CD276/CD155 triple knock-out (T-KO) human induced pluripotent stem cells (iPSC). Each iPSC line was differentiated into β-cells (iBeta), then transplanted in the hindlimb muscles of hIL-15 NOG mice humanized with donor-derived NK cells. Survival of iBeta was evaluated up to 8 months by in vivo imaging system. Functionality was assayed following human c-peptide and glycemia measurements after alloxan-induced diabetes. Strikingly, the T-KO iBeta successfully escaped the chronic NK-mediated allogeneic response in vivo as proved by only a slight reduction of bioluminescence signal during the first days upon transplantation, comparable to the WT counterpart. Conversely, MHC-I-/- iBeta were quickly recognized and rejected by NK cells. Furthermore, T-KO iBeta long-term persisted in vivo (240 days for T-KO vs 10±6 days for MHC-I-/, p<0.0001) and properly maintained their endocrine function in the 60% of transplanted mice, as confirmed by the c-peptide levels and the ability to restore normal blood glucose after diabetes induction. Of note, both MRI and histopathological analysis excluded malignant tumor formations, whereas the perfusion evaluation revealed a significant re-vascularization of the graft area at 8 months after transplantation. We proposed the genetic manipulation of the NK activating ligands as a novel strategy to make MHC-I-null grafts invisible to human NK cells in vivo, offering a new cell therapy-based approach in T1D treatment.