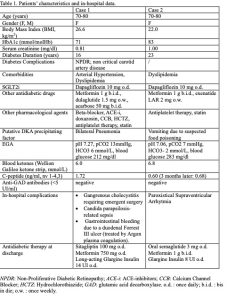
SGLT2i diabetic ketoacidosis (DKA) was thought to be a rare complication observed in diabetic patients treated with sglt2 inhibitors. However, with the increasing popularity of this class of medications, its incidence is increasing. This finding emphasizes the need for educating patients and clinicians to promptly identify precipitating factors, providing them with a sick-day management plan. Notably, ketoacidosis was also recently described in non diabetic patients after the introduction of SGLT2i for heart failure. Below we describe 2 cases of DKA associated with the use of SGLT2i, identified and managed in our ward in 2023. Patients’ salient characteristics and in-hospital data are summarized in Table 1. The first case describes a woman admitted to our ward for non-Covid pneumonia. Dapagliflozin had been prescribed one year before in order to improve glycemic control; along with the prescription, the patient was provided information on the acute situations requiring SGLT2i temporary discontinuation. At admission, the patients was slightly lethargic so an arterial emogas analysis (EGA) was performed, revealing severe DKA. Dapagliflozin and other antidiabetic drugs were stopped; i.v. fluids and i.v. insulin-dextrose infusion were administered until the resolution of acidosis. She then suffered multiple complications during her hospital stay. The second scenario describes a woman admitted for vomiting that had started three days before. Dapagliflozin had been prescribed in another Diabetic Clinic 6 months earlier, and was stopped upon Diabetologic consultation in our E.R. The severe DKA required first care in the Intensive Care Unit, and once stable she was admitted to our ward. I.v. fluids, insulin infusion, i.v. bicarbonate therapy and then insulin-dextrose infusion were administered until the resolution of acidosis. In neither case a significantly elevated lactic acid level was observed. The answer to our opening question is “We definitively need to do more!”. It is not enough to inform patients on the at-risk situations requiring discontinuation only at first prescription, but it is mandatory to develop continuous education programs in order to empower both patients and clinicians to recognize possible red flags and manage at-risk situations safely. Moreover, our efforts should be addressed to identify patients at risk and precipitating factors, that might be milder than previously observed.