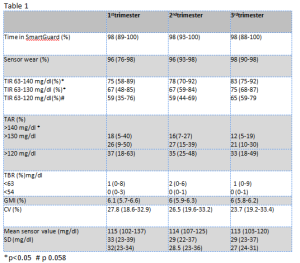
Introduction: Recommended pregnancy specific Time in Range (TIR) 63-140 mg/dl is the result of expert consensus, without strong evidences supporting a significant impact on neonatal complications. Physiological glycaemic values during pregnancy are lower than the most recommended targets for diabetic women (Italian recommendations suggest fasting <90 mg/dl, 1 h postprandial <130 mg/dl, 2 h postprandial <120 mg/dl). Lately, Time in Tight Range (TITR) 70-140 mg/dl has been proposed in non pregnant adults to better represent normal glycaemic profiles, but the is no clear evidence about the target rate (TITR>50% as been proposed, corresponding to Hba1c<6.5%). A recent study on Medtronic 780G pump in non pregnant subjects showed that, with optimal setting, TITR was 57% in adults. There is no data about a pregnancy specific TITR. Patients and methods: We collected retrospective data about 2 hypothetic pregnancy specific TITR (63-120 and 63-130 mg/dl) from 11 type 1 diabetes patients, who were on HCL pump system (Medtronic MiniMed™ 780G), with Glucose Target 100 mg/dl and Active Insulin Time 2 hours, from preconceptional phase until delivery. To by-pass the “safe meal bolus” feature (that adjusts down the bolus amount if the algorithm predicts low glycaemia after meal) and prevent the consequent postprandial hyperglycaemia, women were asked to add an empirical percentage of carbs, not really assumed, to the real amount of carbs, to maintain 1 hour postprandial glycaemic target ≤140 mg/dl and, if late hypoglycaemic trend occurred, to use temporary target 150 mg/dl for 1-2 hours, starting from 60 minutes after meal bolus. Results: All data are reported as median (min-max). Table 1 shows patients’ metrics throughout all trimesters of pregnancy. TITR 63-130 mg/dl is approximately 90% of TIR and TITR 63-120 78% of TIR. All TIR and TITR improve from 1st to 3rd trimester, without significant changes in TBR. TAR>140 reduces more compared to TAR>120 and >130 mg/dl, showing that the improvement in TIR is mostly related to a higher TITR. Conclusions: In this patients, Medtronic 780G helped improving glycaemic control during pregnancy; despite the blood glucose target set of this HCL system is off label. A tighter glycaemic range seems to be achievable, but more studies are necessary to prove a positive impact on maternal-neonatal outcome; moreover it should be investigated whether the risk of hypoglycemia increases when aiming for lower targets.