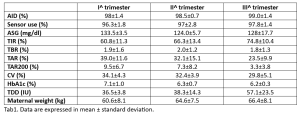
Background and aims: Most commercially available automated insulin delivery (AID) systems are not approved for use in pregnancy. In our clinic, however, 9 women with T1D were offered the use of AID (Tandem t:slim X2 or Minimed 780G insulin pump) during pregnancy and delivery and agreed to it by signing the consent form. This abstract reports the AID treatment outcomes. Materials and methods: Integrated insulin pump data were analyzed from data-sharing portals. For each pregnancy trimester we collected: glucose time-in-range (TIR), time-below-range (TBR), time-above-range (TAR), time above 200 mg/dl (TAR200), average sensor glucose (ASG), glucose coefficient of variation (CV), time using AID, average total daily insulin dose (TDD), HbA1c. Anthropometric data, safety and pregnancy outcomes were also evaluated. Results: We report data from 9 T1D women (mean age 33.8±4.1, diabetes duration 14.2±6.1 years) followed at the Diabetes, Pregnancy, and Technology outpatient clinic in Pescara using AID during pregnancy. The AID mode was used for more than 96% of the time throughout the pregnancy. HbA1c, CV, TIR, TAR and TAR200 improved from the first to the third trimesters. Seven participants achieved a pregnancy TIR target of >70% by the third trimester. TBR remained stable during pregnancy, while TDD showed, as expected, a progressive increase (Tab1). Glycemic profiles remained stable during delivery. The median gestational week at delivery was 37, TIR at delivery 69.7±15.7%, birthweight 3326±460 g, length 50.4±2.3 cm, head circumference 34.7±1.3 cm, Apgar score 1’ and 5’ 7.9±0.7 and 8.7±0.5, respectively. Two women experienced emergency C-section and 2 preterm deliveries. No maternal diabetic ketoacidosis or severe hypoglycemic episodes occurred. Three children had neonatal hyperbilirubinemia, 3 had respiratory distress and 2 experienced isolated low blood sugar requiring treatment. In addition, one case of hypertrophy of the interventricular septum and one ventricular septal defect were reported. Conclusions: Supervised AID use during pregnancy in T1DM, though not currently approved in Italy, resulted in an improved glycemic profile throughout pregnancy and delivery. The findings imply potential benefits, encouraging the use of this system during pregnancy.